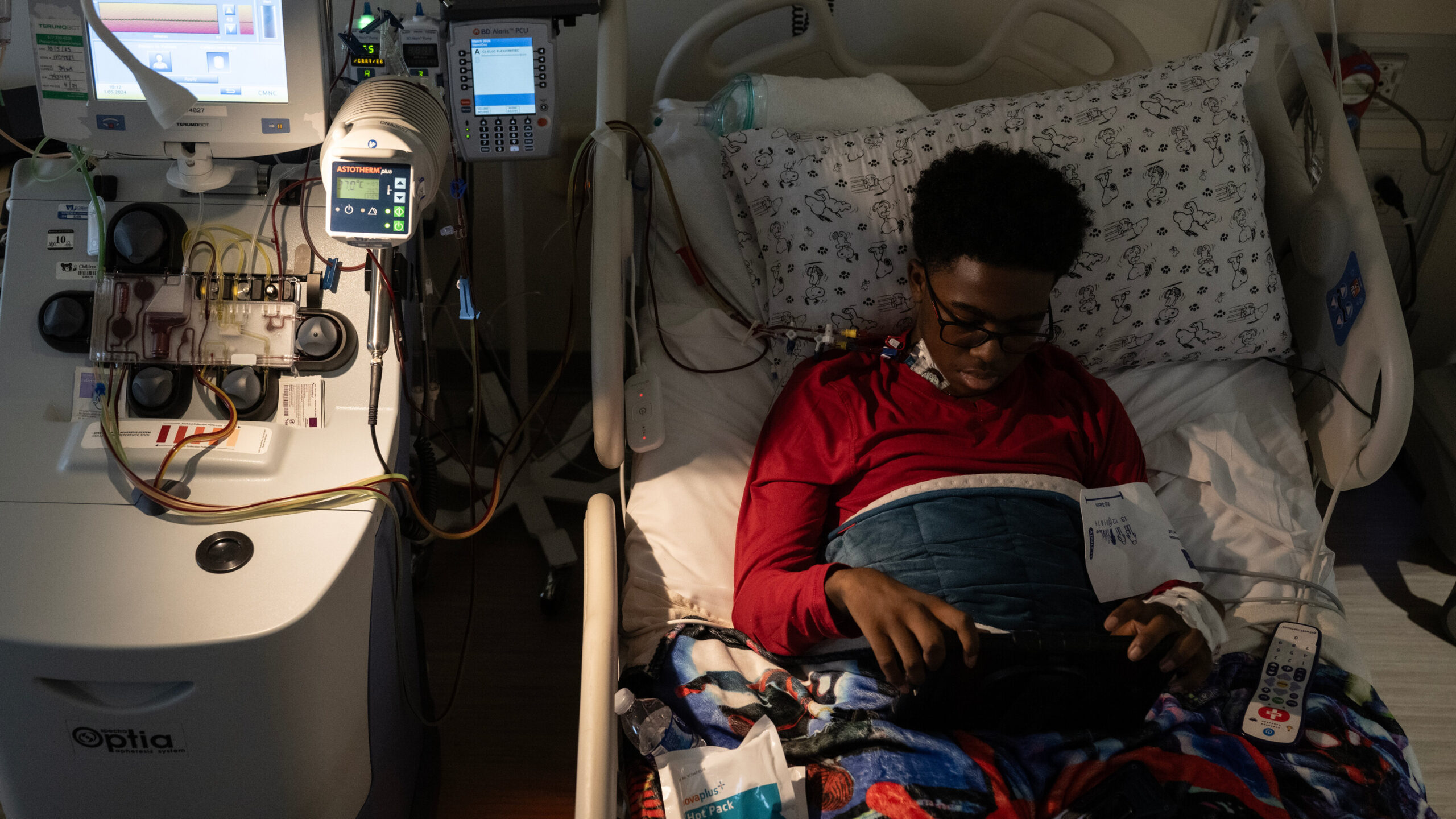
In the Washington, D.C. area, a 12-year-old boy named Kendric Cromer faces an arduous journey ahead as he battles sickle cell disease. His recent initiation into a groundbreaking gene therapy marks a historic moment, offering hope not just for him but for thousands of others affected by the condition in the United States.
For Kendric, living with sickle cell has meant enduring constant pain and limitations on everyday activities like biking or playing soccer. But with the prospect of this new treatment, he dares to dream of a life without the debilitating effects of the disease.
The therapy, authorized by the FDA and offered by companies like Bluebird Bio, holds promise for those with sickle cell disease. Kendric’s case marks the first commercial use of this gene therapy, a significant milestone in medical history.
The process involves a series of intricate steps. Kendric’s journey began with the extraction of his bone marrow stem cells at Children’s National Hospital in Washington. These cells will undergo genetic modification in a specialized lab, aiming to correct the mutated genes responsible for sickle cell disease.
However, the road to recovery is far from easy. The treatment process is lengthy and demanding, requiring months of preparation and intensive care. Only a limited number of patients can be treated each year due to the complexity and resource-intensive nature of the procedure.
Despite the challenges and the hefty price tag associated with the therapy, patients like Kendric and their families hold onto hope for a better future. For Kendric, the possibility of being cured means the chance to pursue his dreams, like becoming a geneticist.