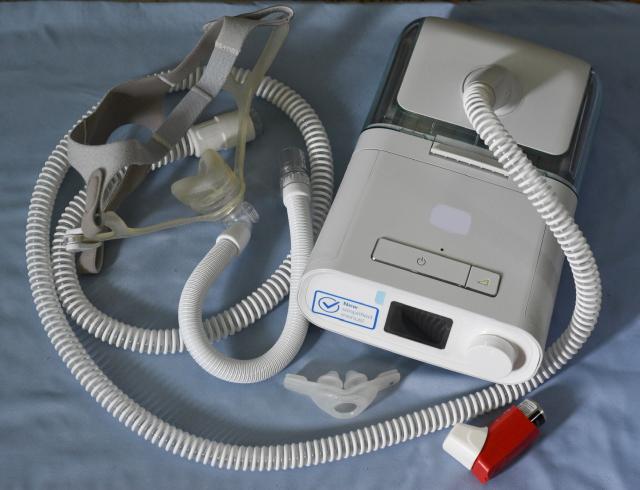
After a prolonged legal battle, Philips has agreed to pay over $1 billion to settle lawsuits filed by thousands of individuals who claim they were harmed by breathing machines that released toxic particles and fumes into their respiratory systems.
The proposed settlement, announced on Monday, will effectively resolve more than 700 lawsuits filed following the 2021 recall of millions of Philips sleep apnea devices and ventilators.
Over 50,000 people are part of the litigation, but it remains uncertain how many will qualify for compensation under the settlement terms. The agreement will be filed in federal court in Pittsburgh.
As part of the settlement, Philips will allocate $25 million to cover the cost of medical monitoring for users concerned about potential long-term health effects, including cancer. Since 2021, the FDA has received over 500 reports of deaths allegedly linked to the machines.
Plaintiffs argue that Philips, which manufactured the devices at two factories near Pittsburgh, should be held responsible for not recalling the machines sooner.
Shawne Thomas, whose husband Rodney died from cancer after using one of the recalled machines, expressed cautious satisfaction with the settlement, stating, “I still don’t have my husband, but it sounds like a good amount of money coming out of their pocket, so it makes me feel a little bit happier.”
An investigation by ProPublica and the Pittsburgh Post-Gazette uncovered that Philips concealed thousands of complaints about an industrial foam used in the machines. This foam could degrade and release harmful material into users’ masks.
Under the settlement terms, Philips did not admit fault or liability. CEO Roy Jakobs described the settlement as providing a “clear path forward for sustainable value creation” in the company’s first-quarter financial report.
Philips had initially warned of serious harm from the degraded foam, but later revised its findings, stating that further testing did not indicate significant health risks.
Last year, Philips agreed to compensate customers for the cost of the defective machines, amounting to over $479 million. However, medical experts believe it could take years to establish links between the machines and specific diseases.
A criminal investigation by the U.S. Department of Justice is ongoing, and the Government Accountability Office is launching an inquiry into the FDA’s oversight of medical device recalls.
Despite criticisms, the FDA has defended its response, stating it acted promptly upon learning of safety concerns in April 2021, shortly before Philips initiated the recall.