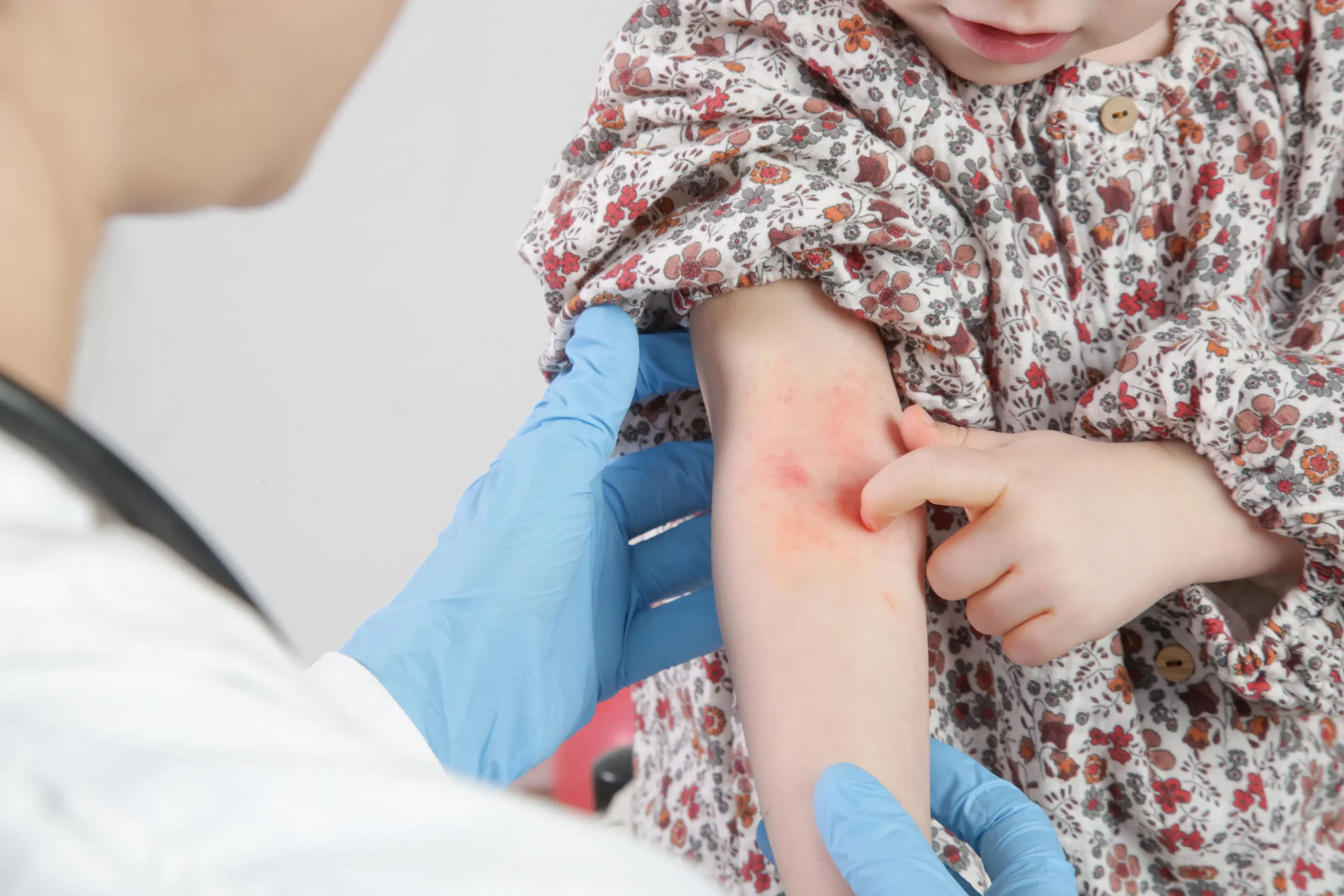
On April 29, 2024, there was some big news from Dermavant Sciences about tapinarof cream, 1%. They announced that the US Food and Drug Administration (FDA) has accepted their request to consider tapinarof cream as a treatment for atopic dermatitis in adults and kids aged 2 and up.
This cream, also known as VTAMA, is already approved for treating plaque psoriasis in adults in the US, and now Dermavant wants to expand its use. They pointed to positive results from some big studies called ADORING 1 and ADORING 2, where tapinarof cream showed promise in treating atopic dermatitis.
They also mentioned that they’ve been studying how kids aged 2-17 respond to the cream. And there’s ongoing research in another study called ADORING 3, which is looking at how well the cream works over a longer period.
Dermavant said they’re hopeful about getting FDA approval by the end of 2024. So, fingers crossed!